|

 Understanding the "sequence-structure-function" relationship of proteins is one of the most challenging problems. For the past two decades, I have been addressing this problem by (i) proposing new predictive schemes to identify the secondary structures of globular and membrane proteins, solvent accessibility, folding rates and kinetics of two-state proteins, and (ii) delineating the important interactions for the stability of proteins and mutants. Further, we have proposed a new method based on direct and indirect readouts for understanding the mechanism of protein-DNA recognition.
Understanding the "sequence-structure-function" relationship of proteins is one of the most challenging problems. For the past two decades, I have been addressing this problem by (i) proposing new predictive schemes to identify the secondary structures of globular and membrane proteins, solvent accessibility, folding rates and kinetics of two-state proteins, and (ii) delineating the important interactions for the stability of proteins and mutants. Further, we have proposed a new method based on direct and indirect readouts for understanding the mechanism of protein-DNA recognition.
Developed surrounding hydrophobicity scales and conformational parameters for globular and membrane proteins and used them successfully for predicting their secondary structures [Gromiha et al. (1997) Prot. Eng. 10, 497-500; Gromiha (1999) Prot. Eng. 12, 557-561]. A web-based prediction server has been developed for identifying membrane spanning beta strands using neural networks [Gromiha et al. (2004) J. Comp. Chem. 25, 762-767; Gromiha et al. (2005) Nucleic Acids Res. 33, W164-167]. Further, we have proposed a statistical methods and machine learning techniques for discriminating outer membrane proteins [Gromiha and Suwa (2005) Bioinformatics 21, 961-968; Gromiha and Suwa (2006) PROTEINS (2006) 63, 1031-1037].
Established a novel method for predicting the real value solvent accessibility (ASA) of proteins, and a web server (RVP-net) has been developed to predict the ASA for any given amino acid sequence. The graphical representation of ASA for each residue in a protein has also been implemented in the server [Ahmad, Gromiha and Sarai (2003) Proteins 50, 629-635; Bioinformatics (2003) 19, 1849-1851]. We are using similar neural network based algorithm for predicting the binding sites of protein-DNA complexes.
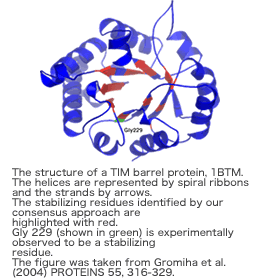
Proposed a novel parameter, long-range order (LRO) from the knowledge of long-range contacts in protein structures. LRO shows a strong correlation with the folding rates of two-state proteins [Gromiha and Selvaraj, (2001) J. Mol. Biol. 310, 27-32]. We have successfully analyzed the relationship between amino acid properties and transition state structures of proteins [Gromiha and Selvaraj (2002) FEBS Lett. 526, 129-134], and the long-range contact network in the folding and stability of TIM barrel proteins [Selvaraj and Gromiha (2003) Biophys. J. 84, 1919-1925; Gromiha et al. (2004) Proteins 55, 316-329]. Further, we have developed statistical models for predicting the folding rates of small, two-state proteins from amino acid sequence [Gromiha (2005) J. Chem. Inf. Model. 45, 494-501; Gromiha et al. (2006) Nucleic Acids Res. In press].
Developed a "Thermodynamic Database for Proteins and Mutants (ProTherm)", which is a collection of more than 16000 data of several thermodynamic parameters, and analyzed the important factors for the stability of proteins upon mutations and for the extreme stability of thermophilic proteins [Gromiha et al. (1999) Nucl. Acids Res. 27, 286-288; (2002) 30, 301-302; Protein Eng. (1999) 12, 549-555; Biophys. Chem. (1999) 82, 51-67].
Analyzed the interaction between amino acid side chains and base pairs and proposed a new method for understanding the mechanism of protein-DNA recognition [Gromiha et al. (2004) J. Mol. Biol. 337, 285-294; Gromiha (2005) J. Biotech. 117, 137-145].

M. Michael Gromiha and M. Suwa (2006). Discrimination of Outer Membrane Proteins using Machine Learning Algorithms. PROTEINS: Struct. Funct. Bioinf. 63, 1031-1037.
M. Michael Gromiha, A. Mary Thangakani and S. Selvaraj (2006) FOLD-RATE: Prediction of Protein Folding Rates from Amino Acid Sequence. Nucleic Acids Res. (in press).
M. Michael Gromiha and M. Suwa (2005). A Simple Statistical Method for Discriminating Outer Membrane Proteins with Better Accuracy. Bioinformatics 21, 961-968.
M. Michael Gromiha, S. Ahmad and M. Suwa (2005) TMBETA-NET: discrimination and prediction of membrane spanning beta-strands in outer membrane proteins. Nucleic Acids Res. 33, W164-167.
M. Michael Gromiha (2005). A Statistical Model for Predicting Protein Folding Rates from Amino Acid Sequence with Structural Class Information. J. Chem. Inf. Model. 45, 494-501.
M. Michael Gromiha and S. Selvaraj (2004) Inter-residue Interactions in Protein Folding and Stability. Prog. Biophys. Mol. Biol. 86, 235-277. (INVITED REVIEW).
M. Michael Gromiha, J.G. Siebers, S. Selvaraj, H. Kono and A. Sarai (2004). Intermolecular and Intramolecular Readout Mechanisms in Protein-DNA Recognition. J. Mol. Biol. 337, 285-294.
M. Michael Gromiha and S. Selvaraj (2001). Comparison between Long-range Interactions and Contact Order in Determining the Folding Rate of Two-state Proteins: Application of Long Range Order to Folding Rate Prediction. J. Mol. Biol. 310, 27-32.
M. Michael Gromiha, J. An, H. Kono, M. Oobatake, H. Uedaira and A. Sarai (1999). ProTherm: Thermodynamic Database for Proteins and Mutants. Nucl. Acids Res. 27, 286-288.
|